Coconut oil is not water-soluble; rather, it is insoluble in water due to the fundamental difference in polarity between the two substances. Water, being a polar solvent, is unable to dissolve coconut oil, which is nonpolar in nature.
Although certain products are marketed as “water-soluble coconut oil,” these are not naturally occurring forms of the oil. Instead, they are modified or specially formulated to disperse in water-based mixtures, and thus do not represent true solubility.
Disclaimer: This information is for educational purposes only and does not replace professional medical or veterinary advice. Always consult a qualified professional before making decisions related to your health or your pet’s health.
Why Coconut Oil Doesn’t Dissolve in Water
Polarity Difference:
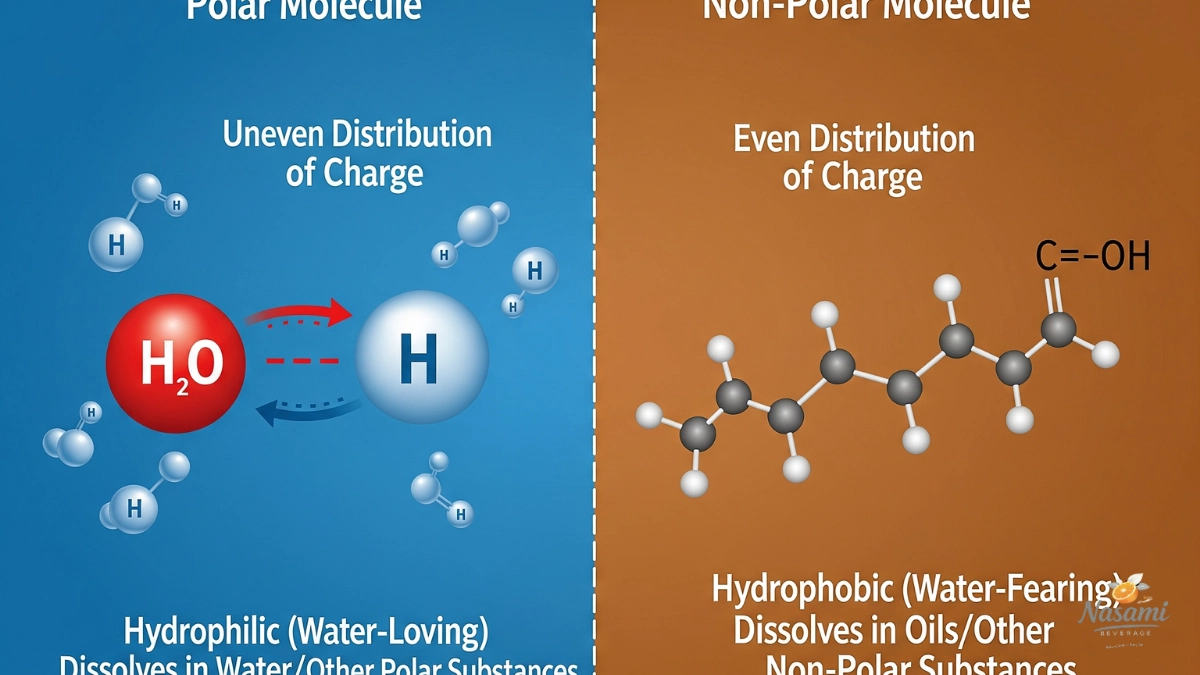
Water molecules are classified as polar because they possess an uneven distribution of electrical charge, with one end carrying a slight positive charge (hydrogen atoms) and the other end carrying a slight negative charge (oxygen atom).
This polarity allows water molecules to attract each other and interact effectively with other polar substances. In contrast, oil molecules, including those found in coconut oil, are nonpolar, meaning they do not have regions of partial positive or negative charges.
As a result, oil molecules do not mix with water molecules, since the polar water molecules are unable to form favorable interactions with nonpolar oil molecules. This fundamental difference in polarity explains why water and coconut oil remain separate when combined.

Like Dissolves Like:
In chemistry, there is a principle often summarized as “like dissolves like.” This means that polar substances have a natural tendency to dissolve in other polar substances, while nonpolar substances are more likely to dissolve in other nonpolar substances.
The reason behind this lies in the types of intermolecular forces that exist between molecules. Polar molecules, such as water, can form strong interactions like hydrogen bonds and dipole-dipole attractions with other polar molecules, which allows them to mix and dissolve effectively.
On the other hand, nonpolar molecules, such as those in coconut oil, rely on weaker London dispersion forces and cannot form strong interactions with polar molecules.
Because water is polar and coconut oil is nonpolar, they are unable to interact in a way that allows them to combine. As a result, when the two are mixed, they remain separate rather than forming a uniform solution.
Density:
Coconut oil has a lower density compared to water, which is why it consistently floats when the two substances are combined. Instead of mixing uniformly with water, coconut oil rises to the surface and forms a distinct, separate layer.
This separation occurs because the lighter, less dense oil molecules cannot sink or disperse into the heavier water molecules. In addition, the lack of compatibility between polar water molecules and nonpolar oil molecules further prevents any dissolution or blending.
As a result, no matter how much the mixture is stirred or shaken, coconut oil will eventually rise and settle on top of the water, maintaining its separate layer. This behavior clearly demonstrates both the density difference and the polarity difference between the two substances.
What “Water-Soluble Coconut Oil” Means
When products are described as “water-soluble coconut oil,” this does not mean that the oil itself has become truly soluble in water. Instead, these products are typically formulated in a way that allows coconut oil to be dispersed in water.
One common method is emulsification, a process that breaks the oil into very small droplets or particles, enabling them to remain temporarily suspended in water without immediately separating.
In some cases, other chemical modifications or the addition of solubilizing agents are used to help the oil blend more effectively with water-based solutions.
These specialized formulations are frequently used in haircare and skincare products, where coconut oil is valued for its moisturizing, nourishing, and protective properties. By dispersing the oil in water, manufacturers can provide the beneficial effects of coconut oil-such as hydration and softness-while avoiding the heavy, greasy texture that pure coconut oil can sometimes leave on the skin or hair.
This makes “water-soluble” versions particularly appealing in lotions, shampoos, conditioners, and sprays, where a lighter and more easily absorbed consistency is desired.

Frequently Asked Questions
Does coconut oil dissolve in hot water?
No, coconut oil does not dissolve in hot water. While heating may melt the coconut oil, causing it to become liquid, it will still remain separate from the water. You will see a layer of oil floating on top of the water.
Can you make coconut oil water soluble?
You can’t change the inherent properties of coconut oil to make it truly water-soluble. However, you can create an emulsion by using an emulsifier, which helps to disperse the oil in water. Some products are marketed as ‘water-soluble coconut oil’ or ‘MCT oil’, which are modified forms of coconut oil that can mix with water.
Is fractionated coconut oil water soluble?
No, fractionated coconut oil is not water-soluble. Fractionated coconut oil is a form of coconut oil where the long-chain fatty acids have been removed, leaving mainly medium-chain triglycerides. It remains an oil and will not dissolve in water.
What can I use to dissolve coconut oil?
Coconut oil is soluble in other oils and organic solvents like alcohol, ether, and chloroform.[1] For cleaning purposes, soap is effective as it can emulsify the oil, allowing it to be washed away with water.
The relationship between coconut oil and water is a clear case of chemical incompatibility. Due to its non-polar molecular structure, coconut oil is hydrophobic and will not dissolve in water. This fundamental property is not a flaw but a defining characteristic that we harness for benefits in the kitchen and in our personal care routines.
While you can’t change its nature, you can use the power of emulsification to bring oil and water together when needed. Understanding this simple science helps you get the most out of this versatile natural product.


